

Description of Bitumen Components
A bitumen’s performance (stiffness, viscosity, low-temperature flexibility, aging) is determined more by its internal makeup than by colour or simple grade numbers. Two samples with the same penetration value may behave differently in service if their chemical fractions differ.
The Main Classified Bitumen Components
Bitumen components are classified into four classes of compounds:
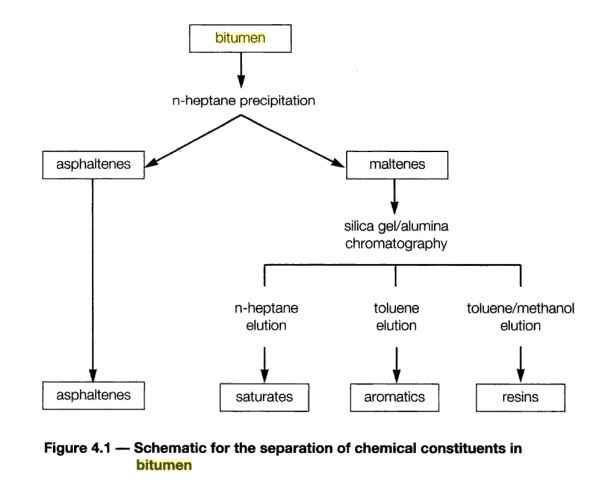
- saturates: Saturates are the lightest, oil-like hydrocarbons. They lower viscosity and improve low-temperature behaviour.
- Naphthenic aromatics: consisting of partially hydrogenated polycyclic aromatic compounds.
- Polar aromatics: consisting of high molecular weight phenols and carboxylic acids
- Asphaltenes: Asphaltenes are the heaviest, most polar fraction. They are high–molecular-weight molecules that provide structural stiffness and contribute to rutting resistance at high temperatures.
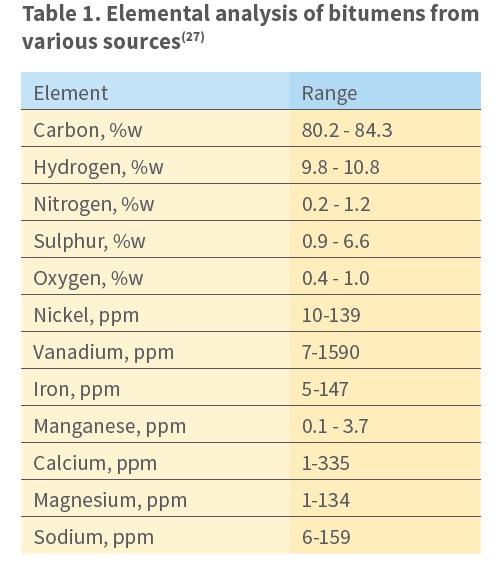
The naphthenic aromatics and polar aromatics are typically the majority components. Additionally, most natural bitumen contains organosulfur compounds, resulting in an overall sulfur content of up to 4%. Nickel and vanadium are found in the < 10 ppm level, as is typical of some petroleum.
Which Bitumen Products Are Manufactured from These Components?
Based on the balance of asphaltenes, resins, aromatics, and saturates, refineries manufacture different penetration-grade bitumen products. Among the most widely used are Bitumen 60/70 and Bitumen 80/100.
Physical Properties of Bitumen
Bitumen is thermoplastic solids or semi-solids at ambient temperature, i.e. they soften as the temperature increases and harden as the temperature decreases. At elevated temperature they behave as Newtonian liquids, the viscosity reducing with increasing temperature. This is the reason that bitumen must be heated for handling and application in their intended use.
The properties of the substances manufactured in the refinery can also be modified for specific end-uses by modifiers described in section 4. The physical properties of the bitumen not only determine the suitability for a given application but also define the conditions under which the product must be handled in order to enable the product to be placed in the structure in which it is to be used.
Physical properties include how bitumen behaves. Here are the main ones:
- Viscosity : means how thick or thin bitumen.
- Penetration: measures how soft or hard bitumen.
- Softening Point: what temperature bitumen becomes soft
- Ductility: shows how much bitumen can stretch before breaking.
- Flash Point: the temperature where bitumen catches fire.
How Temperature Affects Bitumen?
Bitumen behaves differently at different temperatures. In hot weather, it becomes soft. In cold weather, it becomes hard. Good bitumen should not change too much when temperature changes. Scientists measure this change using a method called Penetration Index (PI). When bitumen gets old and reacts with air (oxidation), it becomes more stable and its PI value increases.
This is why PI helps us understand how oxidized the bitumen is. Now, let’s talk about what is inside bitumen. Bitumen contains sulfur (1-7%), which exists in different chemical forms. It also has small amounts of nitrogen, oxygen, and metals. These elements sit inside special ring-shaped structures within the bitumen. Understanding these components helps engineers choose the right bitumen for roads and other projects.
Chemical Properties of Bitumen
Chemical properties means what bitumen is made of at the molecular level.
Elemental Composition
Bitumen functionality relates to how molecules interact with each other and/or with other materials, e.g. aggregate surfaces and water. The content of sulfur, nitrogen, oxygen, and metals in some molecules makes them slightly polar
Chemical Molecular Weight in Bitumen Components
The manufacturing processes for bitumen that are described in section 3 involve the removal of lighter components to leave behind relatively high molecular weight, low volatility compounds. The resulting products all are generally solid or semi-solid materials at ambient temperature and they soften as the temperature increases.
Chemical Characterization of Bitumen
Bitumen is a viscoelastic material; therefore chemical polarity is an important property to measure. The most polar components create structural components which give bitumen stiffness (modulus) properties. Whereas the least polar components give asphalt its flexibility and low-temperature properties, the intermediate polarity components in bitumen compatibilized the least and most polar components.
Since bitumen contains a continuous range of molecules it is impractical to analyze each individual compound. Common practice is, therefore, to divide bitumen into four broad, increasingly polar fractions: saturates, aromatics, resins, and asphaltenes (SARA).
